The atrial fibrillation device market is experiencing significant growth, projected to increase from USD 7,795.23 million in 2023 to USD 19,495.23 million by 2032, representing a compound annual growth rate (CAGR) of 9.96%. Atrial fibrillation (AF) is a common cardiac arrhythmia that affects millions worldwide. Characterized by irregular and often rapid heart rate, AF can lead to serious complications such as stroke and heart failure. The increasing prevalence of AF has spurred significant advancements in medical technology, leading to the development of various atrial fibrillation devices. This article explores the current trends, growth drivers, and future outlook of the atrial fibrillation devices market.
Browse the full report at https://www.credenceresearch.com/report/atrial-fibrillation-devices-market
Market Overview
The atrial fibrillation devices market encompasses a range of products designed for the diagnosis, monitoring, and treatment of AF. These devices include diagnostic tools like electrocardiograms (ECGs) and Holter monitors, as well as treatment devices such as catheter ablation systems, pacemakers, and implantable cardioverter-defibrillators (ICDs). According to recent market analyses, the global atrial fibrillation devices market is experiencing robust growth, driven by technological advancements, rising AF prevalence, and increased awareness among patients and healthcare providers.
Key Market Trends
- Technological Advancements: Innovations in medical technology have led to the development of more efficient and minimally invasive devices. For instance, catheter ablation systems have seen significant improvements, allowing for more precise and effective treatment of AF. Additionally, advancements in wearable technology have enabled continuous monitoring of heart rhythms, facilitating early detection and intervention.
- Integration of Artificial Intelligence: The incorporation of artificial intelligence (AI) in AF devices has revolutionized the market. AI algorithms can analyze large sets of data from wearable devices and ECGs to identify irregular heart rhythms with high accuracy. This has improved diagnostic capabilities and enabled personalized treatment plans.
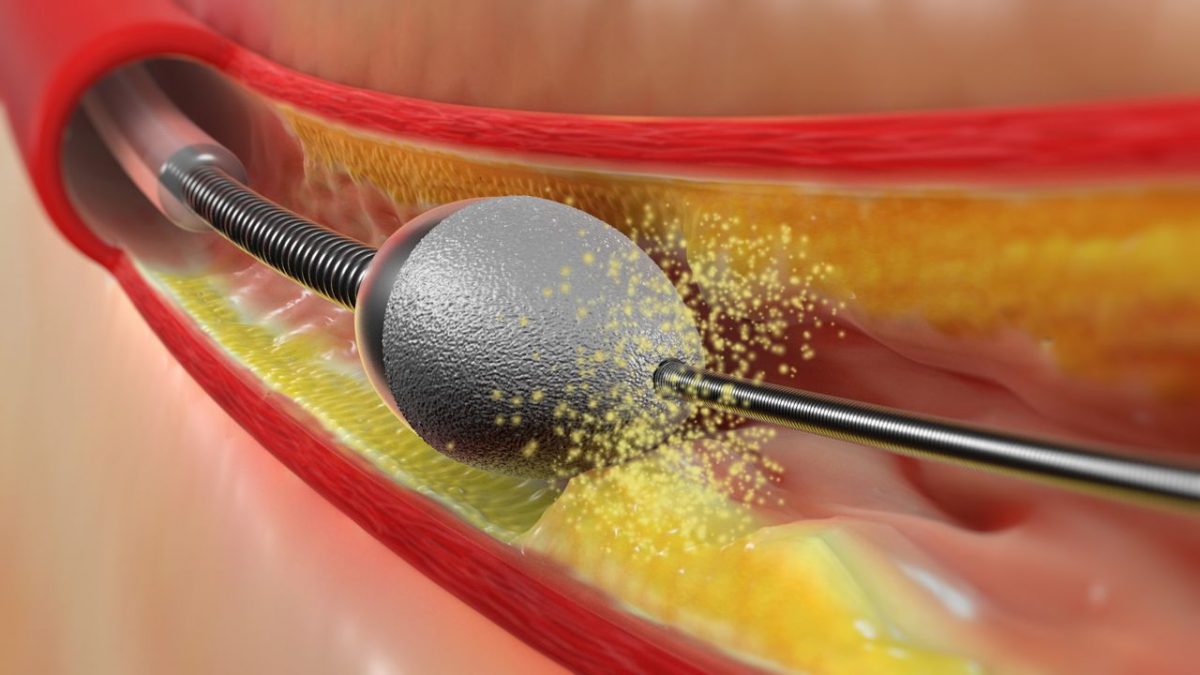
- Increasing Adoption of Catheter Ablation: Catheter ablation is becoming a preferred treatment option for AF due to its high success rate and minimally invasive nature. The introduction of cryoablation and radiofrequency ablation technologies has further enhanced the efficacy and safety of this procedure.
- Rising Demand for Wearable Devices: Wearable devices such as smartwatches and fitness trackers equipped with ECG monitoring capabilities have gained popularity. These devices provide real-time data and have made it easier for individuals to monitor their heart health, leading to earlier diagnosis and treatment of AF.
Growth Drivers
- Growing Prevalence of Atrial Fibrillation: The increasing incidence of AF, particularly among the aging population, is a major driver of market growth. According to the American Heart Association, the prevalence of AF is expected to double by 2050, creating a substantial demand for effective diagnostic and treatment solutions.
- Advancements in Healthcare Infrastructure: Improved healthcare infrastructure, especially in developing regions, has facilitated better access to advanced AF devices. Governments and private organizations are investing in healthcare facilities and technologies, further propelling market growth.
- Favorable Reimbursement Policies: In many countries, favorable reimbursement policies for AF treatments have encouraged the adoption of advanced devices. Insurance coverage for procedures like catheter ablation and the implantation of pacemakers and ICDs has made these treatments more accessible to patients.
- Awareness Campaigns and Preventive Healthcare: Increased awareness about the risks associated with untreated AF and the benefits of early diagnosis and treatment has led to higher demand for AF devices. Preventive healthcare initiatives and educational campaigns by healthcare organizations have played a crucial role in this regard.
Future Outlook
The future of the atrial fibrillation devices market looks promising, with continuous innovations and increasing investments in research and development. Key players in the market are focusing on developing next-generation devices that offer better accuracy, ease of use, and patient comfort. The integration of telemedicine and remote monitoring technologies is expected to further enhance the management of AF, enabling healthcare providers to offer personalized and timely care.
Moreover, the expanding healthcare infrastructure in emerging economies presents significant growth opportunities. As awareness and accessibility to advanced medical technologies improve, the adoption of AF devices in these regions is likely to rise.
Key player:
- AtriCure Inc.
- Boehringer Ingelheim GmbH
- Boston Scientific Corporation
- Bristol- Myers Squibb Corporation
- Cardio Focus Inc.
- Sanofi Aventis
- Biosense Webster Inc.
- Endoscopic Technologies Inc.
- Abbott (St. Jude Medical Inc.)
- Johnsons & Johnson
Segments:
By Treatment type
- Pharmacological Treatment
- Anti-arrhythmic Drugs
- Anticoagulant Drugs
- Non-Pharmacological Treatment
- Catheter Ablation
- Radiofrequency
- HIFU
- Cryoablation
- Microwave
- Laser
- Maze Surgery
- Electric Cardioversion
By End-Use
- Hospitals
- Specialty Clinics
- Others
By Regional
- North America
- US.
- Canada
- Europe
- UK.
- Germany
- France
- Italy
- Spain
- Denmark
- Sweden
- Norway
- Asia Pacific
- Japan
- China
- India
- South Korea
- Australia
- Thailand
- Latin America
- Brazil
- Mexico
- Argentina
- Middle East & Africa
- South Africa
- Saudi Arabia
- UAE
- Kuwait
About Us:
Credence Research is committed to employee well-being and productivity. Following the COVID-19 pandemic, we have implemented a permanent work-from-home policy for all employees.
Contact:
Credence Research
Please contact us at +91 6232 49 3207
Email: sales@credenceresearch.com